Sun Pharmaceutical Industries, Inc. Voluntarily Recalls Three Batches of Kroger Brand Acetaminophen in the U.S. Due to Failure to Meet Child Resistant Packaging Requirement
- Recall involves the Kroger brand acetaminophen over-the-counter drug, which is manufactured by Sun Pharmaceutical Industries, Inc.
- Voluntary recall issued because the packaging of the product does not meet U.S. child-resistant packaging requirements.
- There are no quality or safety issues with the acetaminophen tablets for their intended use. Consumers should immediately store the recalled products in a safe location out of reach and sight of children.
- Contact Kroger at 1-800-576-4377 (1-800-KRO-GERS) from Monday through Friday from 7 am-12 midnight ET, Saturday and Sunday 7 am-9:30 pm ET; online at https://www.kroger.com/i/recall-alerts for information on how to properly dispose the product and receive a full refund.
Product Photos

Recalled Kroger Acetaminophen, 650 mg extended-release caplets, 100 count bottle

Location of batch number on bottle label of recalled product
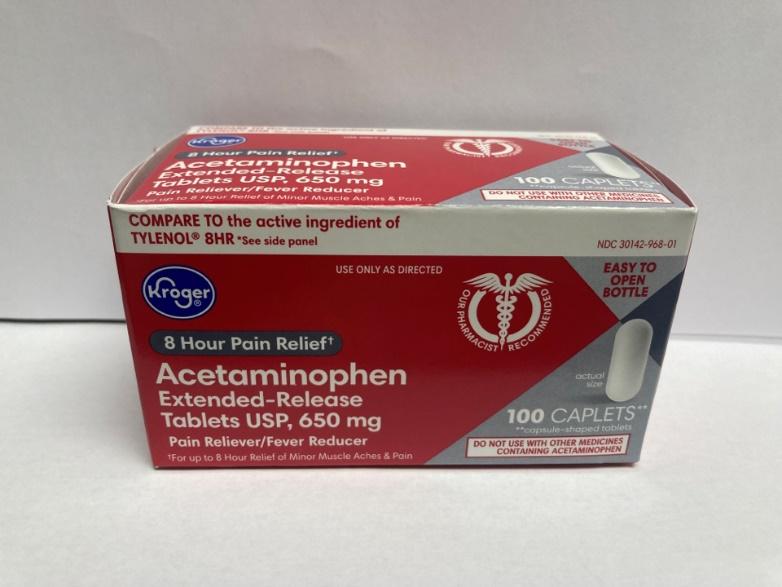
Packaging of recalled product

Location of UPC code on packaging of recalled product
